Novel antifungal shows early promise against Candida auris
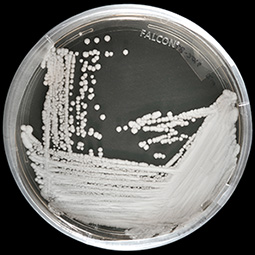
Biotechnology company Scynexis, Inc., is reporting early but promising results from a phase 3 trial of a novel drug for treating invasive Candida aurisinfections.
The company will present the results from the first two case studies in the CARES trial at the upcoming European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Amsterdam. The single-arm trial is evaluating the efficacy and safety of oral ibrexafungerp in patients with candidiasis caused by C auris, a multidrug-resistant fungus that has triggered deadly outbreaks in healthcare facilities around the world, with mortality rates as high as 60%.
AMR NEWS
Your Biweekly Source for Global AMR Insights!
Stay informed with the essential newsletter that brings together all the latest One Health news on antimicrobial resistance. Delivered straight to your inbox every two weeks, AMR NEWS provides a curated selection of international insights, key publications, and the latest updates in the fight against AMR.
Don’t miss out on staying ahead in the global AMR movement—subscribe now!